Documenting implants in the operating room
The client came to us wanting to build a better solution for documenting how high-end medical supplies are used. For medical devices like joint and spine replacements, a single procedure usually consumes thousands and sometimes tens of thousands of dollars worth of parts, so any slip-up could be the difference between solvency and bankruptcy for a troubled hospital.

There are several factors that contribute to a fragmented process:
- frequent changes in technology lead to frequent changes in the supplies - hospital supply chain management software can't keep up with new part numbers, and (potentially) even surgeons aren't the experts on how parts are to be used.
- since supplies are so expensive, hospitals don't want to bear the accounting cost of owning them while they await use. Instead, they invite supplier representatives to bring the supplies onsite.
- at the time of this study, incomplete and unimplemented Electronic Health Record technology ensured a complicated documentation process even within the hospital's own systems.
- the complexity of healthcare pricing creates the possibility of conflicting interests between hospitals, insurers, and suppliers.

Our client wanted to use its dominant position in the healthcare supply chain management industry to bring some order to the situation, and they asked for our help to ensure that hospital staff would be onboard with the solution.
We started with background secondary research in order to create some shared language and a common starting point for understanding the problem. This also gave our design team a chance to get up to speed with the basics of the hospital context - everything from sterile technique to the difference between payors and insurers.
Even from afar, with very limited documentation, we identified the fundamental issues as being related to the way that information on the packaging gets from the vendor representative to the back room of the hospital. But in order to understand the problem well enough to propose a solution, we would have to see it in more detail.

The biggest challenge of this 8-month project was getting permission to visit the inside of an operating room. There are no technical nor legal reasons to deny access, and obviously improving the technology and processes in the hospital will benefit the whole system. But because there are no specific policies or guidelines for permitting design research, it took many months of networking and building bridges to find an accepting location.
Along the way, we had the opportunity to do a bunch of stakeholder interviews. Pictured above, my teammate Courtney Roberts joined me at the 2011 ACORN conference, where we surveyed and talked to more than 100 circulating nurses, operating room techs, hospital billing coordinators, and even a few surgeons. This background allowed us to maximize our time in the context of use looking for things which stood out, and we heard a few stories of the particular kinds of problems to look for.

When we finally got into the operating room, the primary insights were about the intensity and relative importance of issues we had already heard about, and about how these problems interact with each other in a particular setting. For example, we were impressed with how busy the circulating nurse stays - not only are there a multiplicity of documents which have to be filled out beyond the Electronic Health Record, and getting any emergent items that aren't already staged in the OR - the nurse might also be charged with coordinating which operating rooms will be used for which procedures, and keeping track of which tools are in use. When added to their other responsibilities, it becomes obvious why it's problematic for the circulating nurse to be responsible for the hospital's record of what implants are used. While we didn't observe it, it's easy to see why the circulator may just rely on the vendor rep's account of what happens.

The next steps are the most important from a design research point of view. All this knowledge and insight is useless if we can't bring it back to the client team. We presented the findings in the form of video clips to the client team, and summarized our recommendations for the solution in the form of design principles, one of which is shown above.
After a morning session spent reviewing the videos and briefing the team on the business opportunity, we held a hands-on workshop with the client team, including a few role-playing exercises to cement everyone's understanding of the various roles involved in the point of use process.

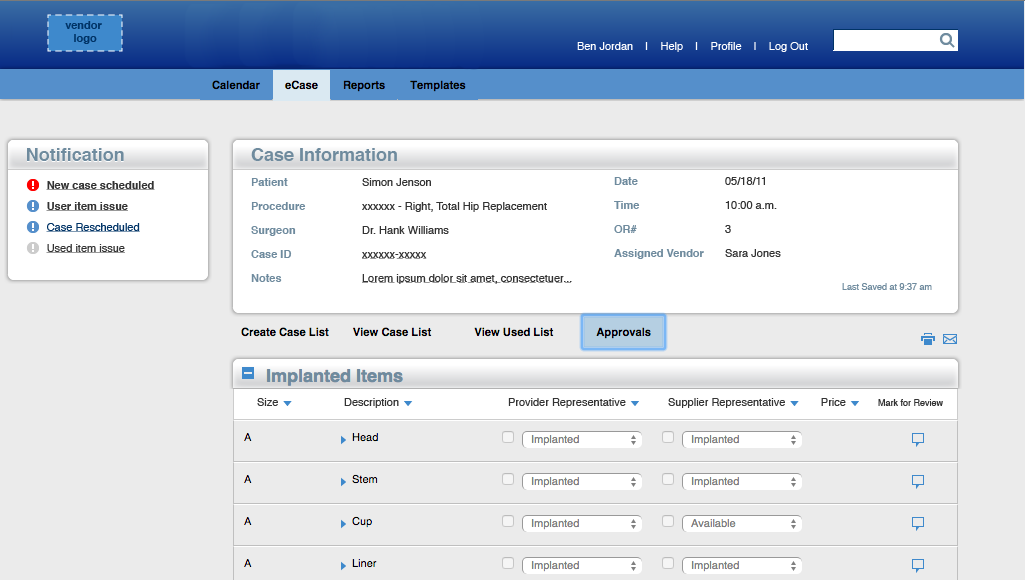
The proposed solution takes this into account by letting the vendor and the circulating nurse communicate with each other through the interface. They're able to keep their own records of what was used, affirm the records input by the other, and leave notes if there are any discrepancies. Please contact me if you're interested in learning more about the next phase of the project - interaction design, prototyping, and user testing.